GLP‑1 Agonists for Weight Loss: Evidence, Benefits, Risks
Key Takeaways
What’s new & why it matters: Modern GLP‑1 agonists (e.g., semaglutide) deliver ~15% average weight loss at 68 weeks in adults with obesity, a step‑change versus legacy medications and a catalyst for rethinking chronic weight management [1].
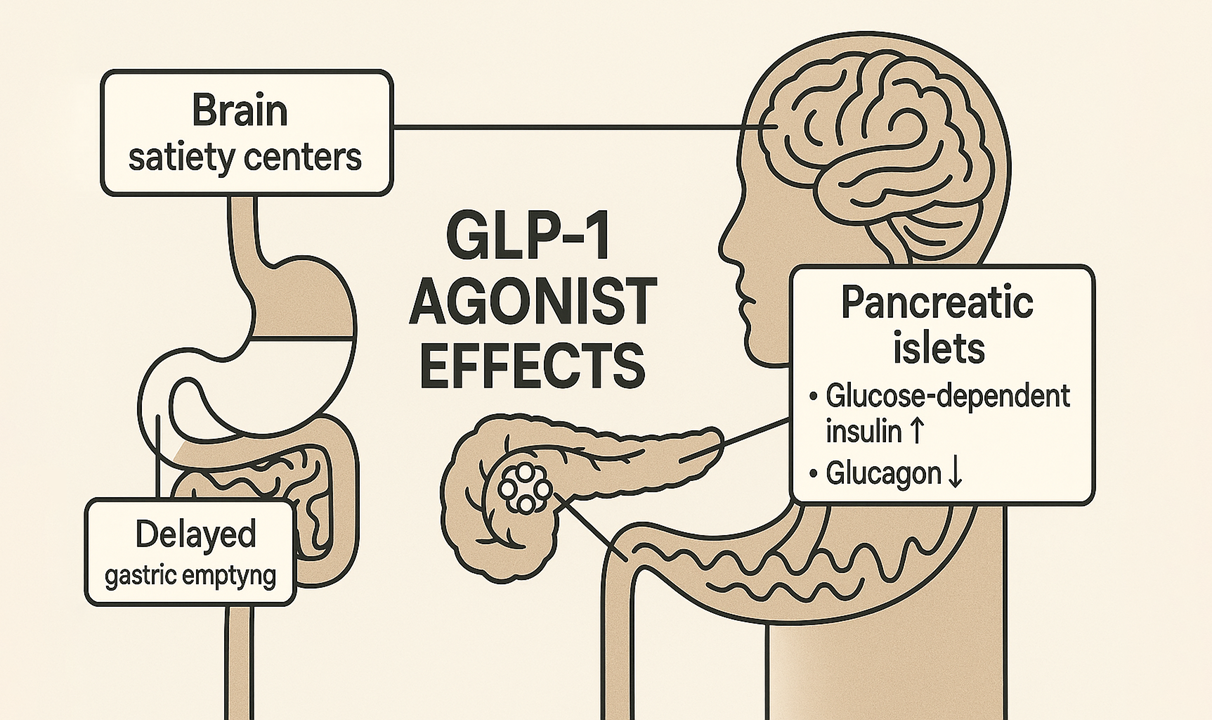
How they work: These medicines activate GLP‑1 receptors to boost insulin when glucose is high, suppress glucagon, slow gastric emptying, and heighten satiety signals—so people feel full with less food [2].
How much weight is typical: Semaglutide 2.4 mg weekly averages ~15% loss at 68 weeks; liraglutide 3.0 mg daily averages ~8% at 56 weeks—both on top of lifestyle support [1, 3].
Safety profile: Gastrointestinal effects (nausea, vomiting, diarrhea) are the most common and usually ease with slow dose titration; pancreatitis signals remain rare and debated; rodent thyroid C‑cell findings have not translated to humans but guide contraindications [1, 4, 7, 8].
Heart and metabolic benefits: Beyond weight, GLP‑1 therapy improves glycemia and, in diabetes, reduces major cardiovascular events; liraglutide cut the composite risk by ~13% in a large outcome trial [5].
Long‑term outlook: Stopping treatment commonly leads to substantial weight regain (≈ two‑thirds of lost weight within a year after semaglutide withdrawal), reinforcing obesity’s chronic, relapsing nature and the need for maintenance strategies [6].
Overview / Background
What GLP‑1 agonists are and why they matter
Glucagon‑like peptide‑1 (GLP‑1) receptor agonists are medications that copy the actions of a gut hormone central to appetite and glucose control. By activating GLP‑1 receptors, these agents stimulate glucose‑dependent insulin secretion, suppress glucagon when glucose is elevated, slow gastric emptying, and modulate satiety pathways in the brain—together reducing caloric intake while improving glycemic control [2, 4].
GLP‑1 medicines act like a built‑in “I’m full” signal while helping the pancreas release insulin only when sugar is high, so people feel satisfied with smaller meals and eat fewer calories [2].
How much weight people lose—and how fast
Two pivotal programs anchor expectations. Daily liraglutide 3.0 mg led to ~8% average weight loss over 56 weeks (−8.4 kg), compared with ~2.6% on placebo in adults with obesity receiving lifestyle support; more participants on liraglutide achieved ≥5% and ≥10% loss than on placebo [3]. Weekly semaglutide 2.4 mg (STEP‑1) delivered ~14.9% mean loss at 68 weeks (−15.3 kg) vs ~2.4% on placebo (−2.6 kg), with many participants reaching ≥10%, ≥15%, and even ≥20% loss [1]. Weight typically declines most in the first 6–8 months and then plateaus; curves in STEP‑1 reached nadir near week 60 and stabilized thereafter under continued therapy [1].
On average, semaglutide reduces body weight by about one‑seventh over a year‑plus; liraglutide achieves about half that—both far beyond older drugs [1, 3].
Mechanisms that support sustained loss
GLP‑1 signaling dampens hedonic and homeostatic drivers of intake while slowing gastric emptying, which together curb caloric consumption. Peripheral effects on pancreatic islets improve post‑prandial glucose handling, and central effects in hypothalamic/brainstem circuits enhance satiety [2, 4].
These drugs help people want—and need—less food at each meal, while smoothing after‑meal blood‑sugar spikes [2].
What else improves beyond weight
In addition to weight loss, GLP‑1 therapy improves glycemic control (including HbA1c) and several cardiometabolic risk markers (lipids, blood pressure) in trials. In type 2 diabetes, liraglutide reduced the composite of cardiovascular death, non‑fatal myocardial infarction, or non‑fatal stroke by ~13% compared with placebo in a large outcome study [5]. Broader reviews report renal and vascular signals consistent with cardiometabolic risk reduction [4].
People not only lose weight; blood sugar control improves and, for some with diabetes, the risk of major heart problems drops [4, 5].
Safety, tolerability, and how to use these medicines wisely
Common effects. Gastrointestinal symptoms—nausea, vomiting, diarrhea, constipation—are the most frequent issues and are dose‑dependent. In STEP‑1, nausea occurred more often with semaglutide than placebo but was generally mild‑to‑moderate and transient; careful dose escalation (slow titration) improves tolerability [1, 4].
Upset stomach is common at first; going slow with dose increases usually makes it manageable [1].
Serious and monitored risks.
Pancreatitis: Rare cases have been reported with GLP‑1 agents; large observational syntheses have not established a clear excess risk versus comparators, but caution is advised—especially in people with prior pancreatitis [7].
Thyroid C‑cell concerns: Rodent studies showed C‑cell tumors with some GLP‑1 agents; human data to date have not demonstrated higher thyroid cancer rates. Nonetheless, drugs in this class are contraindicated in personal/family history of medullary thyroid carcinoma or MEN2, reflecting a precautionary approach [8].
Hypoglycemia: When used alone for weight loss, GLP‑1 agents rarely cause hypoglycemia because insulin release is glucose‑dependent; risk rises if combined with insulin or sulfonylureas, prompting dose adjustments in diabetes [2].
Gallbladder events: Rapid weight loss—by any method—raises gallstone risk; GLP‑1 trials observed small increases in gallbladder‑related events that warrant routine clinical awareness [4].
Serious problems are uncommon; clinicians watch for pancreatitis symptoms, avoid the drugs in certain thyroid cancer histories, and adjust diabetes meds to prevent lows [2, 4, 7, 8].
Who is a candidate—and practical access issues
Regulatory indications for obesity pharmacotherapy center on adults with BMI ≥ 30 kg/m² or BMI ≥ 27 kg/m² with a weight‑related condition, to be used alongside dietary and activity changes—not as stand‑alone therapy [9, 10]. Injection pens and gradual titration are standard; most people adapt to self‑injection with small needles [9]. Real‑world access depends on insurance coverage and prior authorization policies, which vary widely and continue to evolve as obesity is increasingly treated as a chronic disease [9, 10].
These medicines are for adults with obesity (or overweight with health problems) as part of a program that also improves diet and activity; coverage rules differ by insurer [9, 10].
Why stopping often leads to regain
Obesity involves biological defenses of a higher “set point,” including stronger hunger signals and energy‑saving metabolism. In the STEP‑1 withdrawal extension, participants who discontinued semaglutide regained about two‑thirds of the weight they had lost within one year; cardiometabolic improvements also waned as weight returned [6]. Continuous treatment preserved benefits in longer‑term datasets [11].
Turn off the medicine, and the body’s weight‑regain biology tends to turn back on; staying on therapy or planning a maintenance strategy matters [6, 11].
Beyond GLP‑1: what’s coming next
Dual agonists (GLP‑1/GIP): Tirzepatide amplifies satiety and metabolic effects via two receptors; at higher doses it achieved ~20% average loss at 72 weeks in adults with obesity, and is now approved for chronic weight management [12, 10].
Triple agonists: Early trials of agents engaging GLP‑1, GIP, and glucagon receptors (e.g., retatrutide) report >20% mean loss in mid‑stage studies, pointing to further gains but requiring mature outcome and safety data [13].
Oral incretins: Non‑peptide or orally bioavailable agents (e.g., orforglipron) have shown double‑digit weight loss in early studies, potentially broadening access if efficacy and adherence are sustained [13].
Maintenance strategies: Emerging clinician reports and early practice guidance explore slower tapering and behavioral intensification to blunt regain after discontinuation; formal randomized evidence is still limited [14].
Combination therapies: Pairing an amylin analogue with GLP‑1 (e.g., cagrilintide + semaglutide) is under study and has shown additive reductions beyond GLP‑1 alone in early trials [15].
New drugs that hit two or three appetite pathways may help people lose even more weight; researchers are also testing pills and smart ways to keep weight off over time [10, 12–15].
Key numbers at a glance (optional mini‑list)
Semaglutide 2.4 mg weekly: −14.9% mean loss at 68 weeks vs −2.4% placebo [1].
Liraglutide 3.0 mg daily: −8.0% at 56 weeks vs −2.6% placebo [3].
Liraglutide reduced major cardiovascular events by ~13% in high‑risk diabetes [5].
After stopping semaglutide, ≈ two‑thirds of lost weight returned within one year [6].
The newest medicines help many people lose 8–15% (or more) of their weight, can lower heart‑risk events in diabetes, and often must be continued to keep weight off [1, 3, 5, 6].
References
Wilding, J. P. H., Batterham, R. L., Calanna, S., et al. (2021). Once‑weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine, 384(11), 989–1002. https://pubmed.ncbi.nlm.nih.gov/33567185/
Cleveland Clinic. (2023, July 3). GLP‑1 agonists: What they are, how they work & side effects. https://my.clevelandclinic.org/health/treatments/13901-glp-1-agonists
Pi‑Sunyer, X., Astrup, A., Fujioka, K., et al. (2015). A randomized, controlled trial of 3.0 mg of liraglutide in weight management. New England Journal of Medicine, 373(1), 11–22. https://pubmed.ncbi.nlm.nih.gov/26132939/
Wojtara, M., Mazumder, A., Syeda, Y., & Mozgała, N. (2023). Glucagon‑like peptide‑1 receptor agonists for chronic weight management. Advances in Medicine, 2023, Article 9946924. https://pmc.ncbi.nlm.nih.gov/articles/PMC10533252/ https://doi.org/10.1155/2023/9946924
LEADER: Effects of liraglutide on cardiovascular outcomes in patients with type 2 diabetes. Circulation. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.034516
Wilding, J. P. H., Rosenstock, J., & Boden, G. (2022). Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes, Obesity and Metabolism, 24(8), 1553–1564. https://pmc.ncbi.nlm.nih.gov/articles/PMC9542252/ https://doi.org/10.1111/dom.14720
Pancreatitis risk associated with GLP‑1 receptor agonists. [Article; high‑authority review]. https://pmc.ncbi.nlm.nih.gov/articles/PMC11818918/
Pottegård, A., Kristensen, K. B., Reilev, M., et al. (2023). Glucagon‑like peptide‑1 receptor agonist use and risk of thyroid cancer. BMJ, 385, e078225. https://www.bmj.com/content/385/bmj-2023-078225
UCLA Health. (2023, May 24). Semaglutide for weight loss—what you need to know. https://www.uclahealth.org/news/article/semaglutide-weight-loss-what-you-need-know
U.S. Food and Drug Administration. (2023, Nov 8). FDA approves new medication for chronic weight management (tirzepatide press release). https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
Rubino, D., Abrahamsson, N., Davies, M., et al. (2022). Two‑year effects of semaglutide in adults with overweight or obesity. Nature Medicine. https://www.nature.com/articles/s41591-022-02026-4
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., et al. (2022). Tirzepatide once weekly for the treatment of obesity. New England Journal of Medicine, 387(3), 205–216. https://www.nejm.org/doi/full/10.1056/NEJMoa2206038
Nature Editorial Team. (2024). Glucagon‑like peptide‑1 receptor: Mechanisms and advances in therapy. Signal Transduction and Targeted Therapy, 9, 291. https://www.nature.com/articles/s41392-024-01931-z
European Association for the Study of Obesity (EASO). (2024). Is coming off semaglutide slowly the key to preventing weight regain? https://easo.org/is-coming-off-semaglutide-slowly-the-key-to-preventing-weight-regain/
Frias, J. P., et al. (2025). Coadministered cagrilintide and semaglutide in adults with overweight or obesity. New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMoa2502081
About the Author
Harry Negron is the CEO of Jivaro, a writer, and an entrepreneur with a strong foundation in science and technology. He holds a B.S. in Microbiology and Mathematics and a Ph.D. in Biomedical Sciences, with a focus on genetics and neuroscience. He has a track record of innovative projects, from building free apps to launching a top-ranked torrent search engine. His content spans finance, science, health, gaming, and technology. Originally from Puerto Rico and based in Japan since 2018, he leverages his diverse background to share insights and tools aimed at helping others.